
Proteasome Activator PA28γ and p53
p53 was trivially named after the apparent molecular mass it runs on SDS-PAGE, i.e., 53-kilodalton (kDa). Ever since its discovery in 1979 (by several groups simultaneously), this extraordinary phosphoprotein has captured the imagination of life scientists. It is likely the most extensively studied protein in cell biological research.
To underscore its importance, p53 has been called a “cellular gatekeeper” or “the guardian of the genome”. For a long time, researchers believed that it was the universal master switch that defined cancer onset and progression. However, this turned out to be not entirely true. Nonetheless, at least half of all cancer types carry aberrations in the p53 gene (TP53). It also acts as a decision nodule that organizes the cell’s response to stress.
The approach to p53
The consequent result would basically be to “live or let die”, since p53 determines if the cell responds to stress with cell cycle arrest, apoptosis, senescence, DNA repair, or autophagy. Its tumor suppressor function was recognized early on. However, ever-increasing evidence indicates that p53 plays an intricate role in normal cellular metabolism, and is able to act as an oncogene.
Furthermore, p53 not only regulates a variety of essential proteins via various regulatory mechanisms, but its activity and action are also regulated by numerous proteins. As a result, its dysfunction has detrimental consequences in a plethora of signalling and metabolic pathways and, therefore, its key role in malignant transformations is not surprising.
PA28γ and p53
In The Proteasome Activator PA28γ, a Negative Regulator of p53, Is Transcriptionally Up-regulated by p53 published in IJMS, Wan and co-workers unravel an important regulatory mechanism that appears to play a role in maintaining appropriate intracellular levels of p53 by regulating its proteosomal degradation.
The proteasome is a multi-subunit proteolytic complex that consists of a cylindrical catalytic core unit and one or two regulatory units (proteasomal activator), and carries out selective, efficient, and processive hydrolysis of proteins via polymerization of ubiquitin. This ubiquitin–proteasome system controls virtually all basic cellular processes, including signal transduction, cell death, immune responses, metabolism, cell cycle progression, and protein quality control.
The proteosome
The proteosome is activated by specific activators that bind to its regulatory subunits, including PA28 and PA200. There are three PA28 family members, PA28α,β,γ, PA28γ (also called REGγ, 11Sγ, or PSME3). These are implicated in the regulation of cell proliferation, apoptosis, and carcinogenesis. They also predominantly exert their functions in nuclear proteolysis.
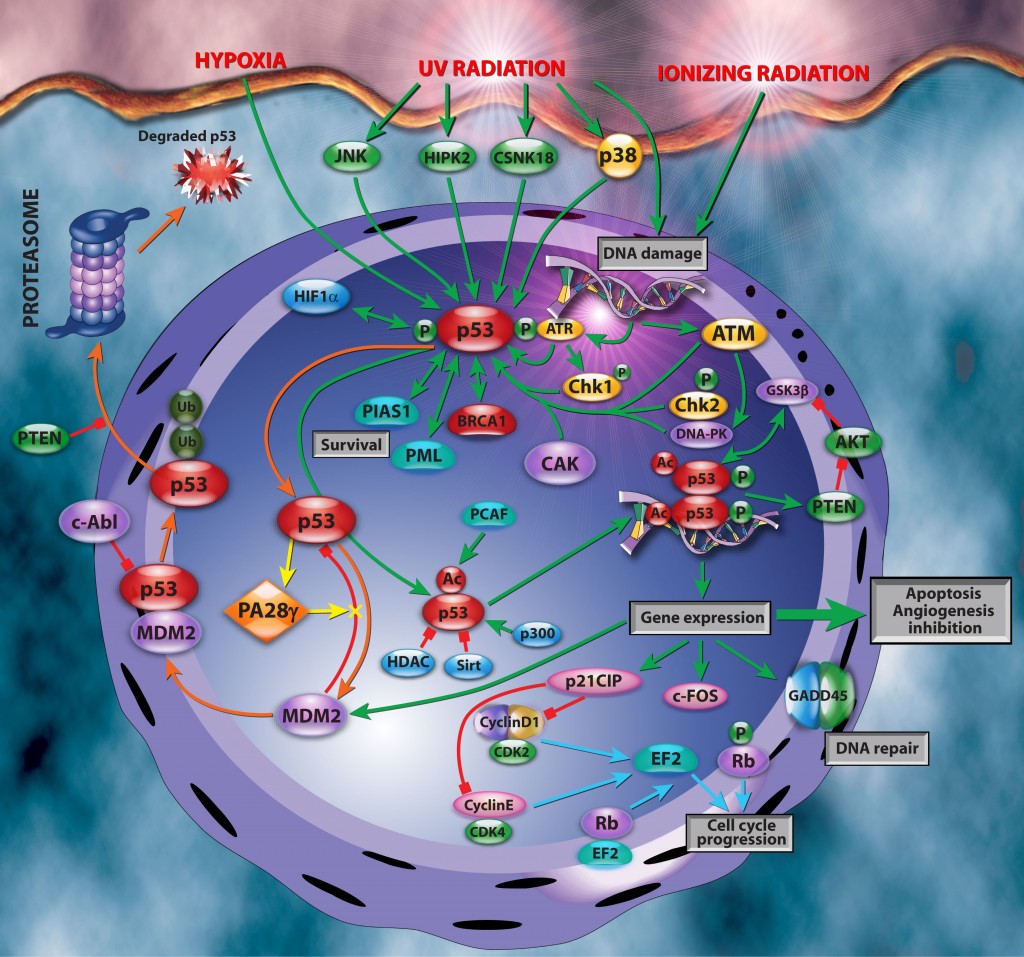
Emerging evidence shows that PA28γ contributes to p53 turnover via MDM2-mediated proteasomal degradation. It does so by facilitating interaction between MDM2 and p53 (figure above). This thereby promotes ubiquitylation and proteasomal degradation of p53. This mechanism inhibits apoptosis after DNA damage by limiting its accumulation (negative regulator) and underscores PA28γ’s involvement in apoptosis and cell proliferation. However, Wan et al. show that this is not a one-way street and that p53 itself is capable of binding to the promoter of PA28γ and up-regulating PA28γ’s expression. Furthermore, this upregulation can be suppressed by PA28γ itself. Thus forming a negative feed-back loop between p53 and PA28γ, similar to MDM2 and p53 (figure above). The authors propose that PA28γ may function as a cofactor of MDM2 in maintaining an appropriate level of p53. This is essential to prevent cancer initiation and progression. It also maintains DNA damage repair and chromosomal stability.
Interactions
This new insight on how p53, MDM2, and PA28γ interact is an important step in understanding how aberrations in this triangular relationship contribute to cancer, and might lead to the development of novel therapeutic strategies. For instance, directed elimination of endogenous PA28γ in cancer cells, perhaps with an interfering antibody or by silencing its expression, would inhibit MDM2-mediated p53 degradation.
Consequently, this increased activity of p53 would stimulate apoptosis; an attractive therapeutic prospect. Additionally, PA28γ might serve as a biomarker in early cancer events. This was recently suggested for colorectal cancer by Chen et al.
Referenced works
Further reading on the subject above can also be found here:
- Levine, A. J., p53, the cellular gatekeeper for growth and division. Cell 1997, 88, (3), 323-31.
- Lane, D. P., Cancer. p53, guardian of the genome. Nature 1992, 358, (6381), 15-6.
- Wan, Z. X.; Yuan, D. M.; Zhuo, Y. M.; Yi, X.; Zhou, J.; Xu, Z. X.; Zhou, J. L., The proteasome activator PA28gamma, a negative regulator of p53, is transcriptionally up-regulated by p53. Int J Mol Sci 2014, 15, (2), 2573-84.
- Tanaka, K., The proteasome: overview of structure and functions. Proc Jpn Acad Ser B Phys Biol Sci 2009, 85, (1), 12-36.
- Mao, I.; Liu, J.; Li, X.; Luo, H., REGgamma, a proteasome activator and beyond? Cell Mol Life Sci 2008, 65, (24), 3971-80.
- Liu, J.; Yu, G.; Zhao, Y.; Zhao, D.; Wang, Y.; Wang, L.; Li, L.; Zeng, Y.; Dang, Y.; Wang, C.; Gao, G.; Long, W.; Lonard, D. M.; Qiao, S.; Tsai, M. J.; Zhang, B.; Luo, H.; Li, X., REGgamma modulates p53 activity by regulating its cellular localization. J Cell Sci 2010, 123, (Pt 23), 4076-84.
- Zhang, Z.; Zhang, R., Proteasome activator PA28 gamma regulates p53 by enhancing its MDM2-mediated degradation. EMBO J 2008, 27, (6), 852-64.
- Chen, D.; Yang, X.; Huang, L.; Chi, P., The expression and clinical significance of PA28 gamma in colorectal cancer. J Investig Med 2013, 61, (8), 1192-6.
Further Reading
We also refer interested readers to the following articles.
- Cajee, U. F.; Hull, R.; Ntwasa, M., Modification by ubiquitin-like proteins: significance in apoptosis and autophagy pathways. Int J Mol Sci 2012, 13, (9), 11804-31.
- Fu, T.; Min, H.; Xu, Y.; Chen, J.; Li, G., Molecular Dynamic Simulation Insights into the Normal State and Restoration of p53 Function. Int J Mol Sci 2012, 13, (8), 9709-40.
- Holmberg Olausson, K.; Nister, M.; Lindstrom, M. S., p53 -Dependent and -Independent Nucleolar Stress Responses. Cells 2012, 1, (4), 774-98.
- Lehman, J. A.; Mayo, L. D., Integration of DNA damage and repair with murine double-minute 2 (mdm2) in tumorigenesis. Int J Mol Sci 2012, 13, (12), 16373-86.
- Lew, Q. J.; Chu, K. L.; Chia, Y. L.; Cheong, N.; Chao, S. H., HEXIM1, a New Player in the p53 Pathway. Cancers (Basel) 2013, 5, (3), 838-56.
- Liu, W.; Lu, X.; He, G.; Gao, X.; Xu, M.; Zhang, J.; Li, M.; Wang, L.; Li, Z.; Luo, C., Protective roles of Gadd45 and MDM2 in blueberry anthocyanins mediated DNA repair of fragmented and non-fragmented DNA damage in UV-irradiated HepG2 cells. Int J Mol Sci 2013, 14, (11), 21447-62.
- Mirzayans, R.; Andrais, B.; Scott, A.; Wang, Y. W.; Murray, D., Ionizing radiation-induced responses in human cells with differing TP53 status. Int J Mol Sci 2013, 14, (11), 22409-35.










