Vaccines for Alzheimer’s Disease
Alzheimer’s Disease (AD) is a type of neurodegenerative disease that can cause dementia. Dementia is an umbrella term for progressive, ageing diseases affecting cognitive functions like memory, reasoning and learning. According to the World Health Organization (WHO), around 55 million people have dementia, with about 50‒60% of these cases caused by AD.
The pathogenesis of AD is complex and multiple factors may cause the development of the disease. AD is characterized by a build-up of two components known as amyloid-beta (Aβ), causing Aβ plaque formation, along with neurofibrillary tangles formed by tau protein accumulation. An accumulation of these components results in the damage and shrinkage of neurons.
There is currently no cure for Alzheimer’s disease, but there are medications which may help ease the symptoms and slow the progression of the disease. For example, cholinesterase inhibitors help improve cell-to-cell communication and selective serotonin reuptake inhibitors are given to patients with AD who experience anxiety or depression.
Vaccines against AD are being continuously researched and may be a potential cure for the disease. However, vaccine candidates are still in development or undergoing clinical trials.
In this article, we will explore studies on vaccine development against AD and the obstacles researchers face in their development. We also discuss monoclonal antibodies as a treatment for early AD.
Amyloid-beta as a target for Alzheimer’s Disease
As AD can be characterised by the accumulation of Aβ plaques in the brain, most vaccine research for AD focuses on targeting this component and its removal from the brain.
Peptide vaccines for Alzheimer’s Disease
The first developments of Aβ vaccines consisted of vaccinating the individual with an Aβ peptide to generate anti-Aβ antibodies. However, anti-Aβ vaccines initially showed adverse side effects during clinical trials. These included the development of meningoencephalitis (inflammation of the meninges and brain) in patients who received the AN1792 vaccine during its phase II trials. This may have been due to the overactivation of helper T cells recruited in response to a specific region of the Aβ peptide.
Ralph Tripp, Editor-in-Chief (EiC) of the Open Access journal Vaccines provides a perspective on the previous and current landscape of AD vaccine research, including the trial of AN-1792:
In theory, vaccination with AN-1792 was thought to induce antibodies against potentially toxic plaques in the brains of AD patients, slowing their cognitive decline. However, these antibodies ended up attacking healthy cells in several patients’ brains, leading to severe cases of brain swelling and the trial’s early termination. A significant issue in vaccine development for AD is inducing an immune response against Aβ (an endogenous protein) that would require breaking immune self-tolerance. Further, it was unclear whether eliciting an immune response against a neuro-protein such as Aβ would be possible or even practical. Moreover, antibody transport from the blood to the brain is limited, as the brain is immune-protected by the blood‒brain barrier (BBB), which prevents the passage of most proteins and small molecules.
Efforts were therefore put into the re-design of the vaccine to ensure safety and tolerability, as well as immunogenicity. This included the development of DNA vaccines which consisted of DNA fragments that coded for Aβ instead.
DNA vaccines for Alzheimer’s Disease
A 2023 review published in Vaccines by Vicidomini et al. summarises current DNA vaccinations for AD and discusses the theory behind immunization against Aβ. In the DNA vaccine, DNA coding for Aβ is present, triggering the production of antibodies against them. Then, Aβ antibodies cross the blood‒brain barrier and enter the central nervous system to reach Aβ -plaques and eliminate them.
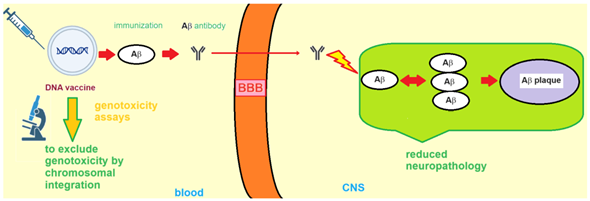
Schematic explaining theory of DNA vaccines targeting Aβ-plaques (https://doi.org/10.3390/vaccines11111706).
Several studies found a reduction in Aβ plaques following the administration of DNA vaccines targeting Aβ in mouse models, without major adverse side effects. Vaccine candidate AV-1959D is a DNA vaccine that is currently in phase 1 clinical trials, which involves testing the safety and tolerability of the vaccine in patients with early AD. The vaccine was developed with an emphasis on Aβ clearance and the prevention of T-cell overactivation, which previously resulted in the development of meningoencephalitis, as discussed.
One of the main challenges of developing vaccines that target Aβ is the safety of the vaccine while inducing a sufficient immune response. This includes preventing the overaction of the immune response, such as excess inflammatory cytokine production and helper T-cell activation.
Previous vaccine candidates have struggled with maintaining a sufficient antibody titre in the body to neutralise Aβ plaques. The biological differences between animal and human models are highlighted here, as studies still struggle to replicate the success of Aβ plaque reduction and safety found in animal models.
According to the review by Vicidomini et al.:
Vaccination might be the most promising approach to reducing the global burden of dementia.
Latest treatment breakthroughs
UB-311 vaccine
Contrary to previous results, a recent breakthrough by pharma company Vaxxinity reported success in phase 2a clinical trials for a re-designed peptide-based Aβ vaccine called UB-311. They reported the safety, tolerability and immunogenicity of the vaccine in patients with mild AD. The results showed a 97% antibody response rate which was maintained at 93% by the end of the study.
This addresses one of the challenges researchers have found—maintaining antibody titre. The study was carried out on 48 participants in Taiwan. However, they found that 14% of the patients treated suffered from microhemorrhages and haemosiderin deposits (accumulation of components from the breakdown of red blood cells).
However, due to the overall success of the vaccine in its safety and generation of a robust immune response, the vaccine will be put forward to enter phase III clinical trials. Here, the safety and effectiveness of the vaccine will be tested on a much larger group of participants.
Protollin vaccine
Protollin is an intranasal vaccine that does not consist of or generate anti-Aβ antibodies, like previous vaccines discussed. Instead, it contains bacterial proteins that activate the innate immune system which targets Aβ plaques in AD patients. The vaccine was developed at Brigham and Women’s Hospital and is currently entering Phase I human clinical trials to test for safety, efficacy and tolerability. Dr. Weiner, leader of the study, states:
…it’s taken nearly 20 years of work in the laboratory to get to this first stage, so it’s a major milestone that we’ve gotten it into Alzheimer’s patients.
Monoclonal antibodies to treat Alzheimer’s disease
Vaccines are a type of active immunotherapy, where the immune response is activated upon its administration. Monoclonal antibodies (mAb) treatment, on the other hand, is a type of passive immunotherapy.
The challenge with using mAb therapy for AD and its current progress is discussed by Ralph Tripp:
Antibody transport from the blood to the brain is limited, as the brain is immune-protected by the blood‒brain barrier (BBB), which prevents the passage of most proteins and small molecules. Additionally, AD is not fully understood. Currently, the industry’s aim is the development and approval of disease-modifying therapies (DMTs) and symptomatic treatments for AD. Two anti-amyloid monoclonal antibodies—aducanumab and Lecanemab—that slow the cognitive decline of AD have been approved, and another—Donanemab—is currently under FDA review. – Ralph Tripp, EiC of Vaccines
As mentioned, there are currently a few approved mAb treatments to help slow the progression of AD.
This includes a drug called Lecanemab. This treatment is currently approved by the U.S. Food and Drug Administration agency for adult patients with AD at early or mild stages of dementia. This approval was given upon evidence of a decrease in specific forms of amyloid plaques, seen in the double-blind phase III clinical trial. Along with Lecanemab, there are other potential mAb candidates to treat early AD, such as Donanemab. This mAb also showed a reduction in amyloid plaques in those with early-stage AD.
The success of mAb therapy development can provide AD vaccine researchers with a wealth of information, especially for the development of vaccines that stimulate innate anti-Aβ antibodies.
As mentioned, approved mAbs for AD have been recommended for people who are in the early stages of the disease. Therefore, diagnostics need to be of utmost importance to ensure that patients can receive the treatment in time before the disease has progressed too much.
Further research
Research on vaccines for the treatment of AD has progressed immensely since the termination of the AN1792 clinical trial in 2005. Re-designed peptide vaccines such as UB-311 show promising results in phase IIa clinical trials. Furthermore, DNA vaccine research is also on the way, with vaccine AV-1959D entering phase I clinical trials. Approved mAb therapy shows success in slowing down the progression of the disease in patients with early-stage AD. The importance here lies in early diagnostics in patients to receive effective treatment at an early enough stage.
Further, the EiC discusses the importance of vaccine research as a means of AD treatment.
The importance of vaccine research for AD is clear. Compared to passive immunotherapies, vaccination is affordable but can target a large population of susceptible people and induce longer-lasting protection than passively administered monoclonal antibodies. Vaccine development must combine satisfactory effectiveness with an acceptable level of safety for patients. Interestingly, nucleic acid-based and peptide vaccines are being explored in the fight against AD, showing promising features. – Ralph Tripp, EiC of Vaccines
Research on AD treatment and vaccines needs to be open to enable efficient dissemination of research findings and hence, treatment progression. Vaccines is an Open Access journal that publishes novel and up-to-date findings in the field of vaccine research.
Access Vaccines if you would like to read more about AD vaccine research, or click here to read more MDPI Open Access research across a range of scientific disciplines.










