
Authorship Guidance: Who to Include and Who to Acknowledge
15,025 is the new record of co-authors listed on a published paper as of March 2025. This type of development raises several authorship concerns regarding the growing number of authors being listed in published articles and brings into question who precisely should be listed as an author.
Being an author of a research paper is not only rewarding but can also be significant for the professional development of academics. Therefore, authors discussing authorship lists and being aware of publication norms can be extremely important for reducing the likelihood of disputes and other post-publication issues.
From a publisher perspective, having clear guidance preempts any potential disagreements and ensures accountability and transparency.
Here, we discuss the general criteria that need to be fulfilled to be considered as an author on a research paper and provide guidance on avoiding disputes and the use of AI.
Who to include as an author?
As researchers try to address complex problems, they rely on collaborations across diverse fields such as medicine, chemistry, physics and engineering. This interdisciplinary approach to science results in an increasingly larger and diverse group of authorship.
Medical research has a longstanding history as a collaborative environment with clinicians working closely with pharmacologists, biologists and statisticians. The International Committee of Medical Journal Editors (ICMJE) provides recommendations regarding best practice and ethical standards for publishing biomedical research. Their guidance is now being adopted by publishers even outside the biomedical field.
The ICMJE defines authorship based on 4 criteria:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
- Drafting the work or reviewing it critically for important intellectual content; AND
- Final approval of the version to be published; AND
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
To be included as an author, all 4 criteria must be met. At the same time, researchers should include authors by giving them the opportunity to contribute to drafting, reviewing or giving final approval.
The ICMJE guidelines are just one of many guidelines, with each discipline creating their own definition based on practices within their field. For example, physics is know for having “mega-authored” content and accounts for 91% of content with 500+ authors per publication.
The Committee on Publication Ethics (COPE) has therefore set a minimum criterion that is common to all definitions and could be applicable across disciplines. According to COPE, authors must fulfill the following:
- Substantial contribution to the work; AND
- Accountability for the work that was done and its presentation in a publication.
First and corresponding authors
The first named author within the authorship list is a coveted position. Future researchers will cite the article by using the first author’s name, thus providing recognition for their contribution. Therefore, the first author is usually considered the primary contributor to the research paper. For example, if a research article is based on the PhD dissertation of a student, that student is typically named as the first author in the manuscript.
The corresponding author is the designated point of contact between the journal editor and the rest of the authors during the peer review process. They will also ensure that all requested documentation is submitted to the journal editor and confirm that all ethical requirements are met.
The role of the corresponding author does not end when the paper is published. They take on the responsibility of answering questions readers may have, providing additional data or details if required and cooperating with the journal team in case post-publication corrections are needed.
Due to the long-term position held, the corresponding author will usually be a senior member of the group that has an overview of the entire project and access to all data generated.
Avoiding authorship disputes
Conversations about authorship should take place as early as possible and any agreement or change throughout the lifecycle of the project should be documented. There are many resources available that can help authors navigate this sensitive topic.
As a starting point, authors can consult the NIH Guidelines for authorship contributions, which is also a recommended resource from COPE.
This guideline breaks down the general types of contributions researchers can make towards a research publication. Each contribution category is then assigned a gradient scale to indicate if authorship is warranted or not.
This document is not precise but can be used to guide discussions amongst the potential authors at the start of a project.
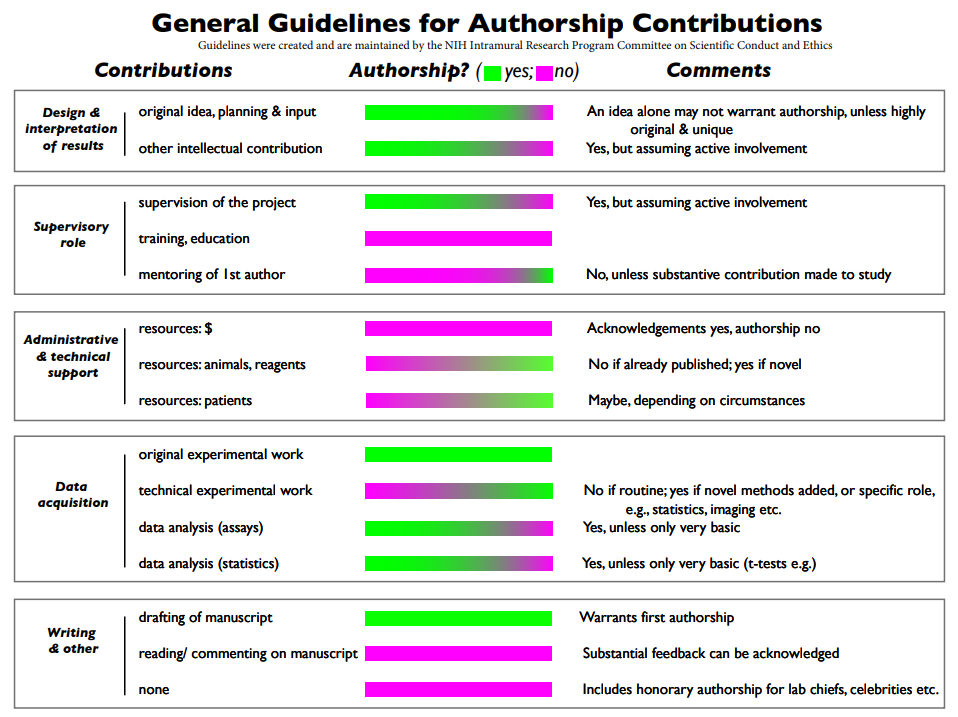 NIH Guidelines on Authorship Contributions.
NIH Guidelines on Authorship Contributions.
Alternatively, a more numerical approach can be taken. Martins et al. developed a tool called the “CalculAuthor” which can be used to create a scoring system to determine the contribution of each author. All collaborators can work together to create the scoring sheet and can self-evaluate their contribution.
By working in a transparent and open way, authors will be able to reach an agreement regarding not only inclusion but also regarding the order of authorship.
How the Acknowledgements section works
In all projects, there may be individuals that facilitated the research or final publication but do not meet the criteria for authorship. For example, they may have donated one of their cell lines or other special materials, helped with technical or language editing, or engaged in a fruitful discussion at the start of the project. Their efforts should be recognized within the Acknowledgements section. Each acknowledged person should be listed together with a brief description of their contribution.
Within the field of medicine, professional medical writers may be employed to assist the team with the communication of their research results. Medical writers have extensive knowledge of ethical publication guidelines and ensure that the authors are aware and adhere to the best publication practices. The medical writer’s contribution should be included in Acknowledgements following the template:
“The authors thank [name and qualifications] of [company, city, country] for providing medical writing support/editorial support [specify and/or expand as appropriate], which was funded by [sponsor, city, country] in accordance with Good Publication Practice (GPP3) guidelines.”
Authors who use artificial intelligence (AI) for assistance with writing must also declare it within the Acknowledgements section. Details of the tools used and how they were used should be included.
Ethical use of generative artificial intelligence
The emergence of generative AI (GenAI) provides many opportunities as well as challenges within scholarly publishing. As authors explore and leverage these tools, it is important to establish best practices to maintain transparency and ensure ethical use.
The International Association of Scientific, Technical & Medical Publishers (STM) released a white paper that includes ethical and practical guidelines for the use of GenAI. According to this guide, the use of GenAI tools by authors for formatting, refining and editing texts is permissible and does not need to be declared.
Learn more about how to use AI tools for editing your paper in our article Can Artificial Intelligence Be Used To Edit Your Paper? here.
Uses beyond these cases must be disclosed as part of the submission and a detailed procedure must be included within the Materials and Methods section. The journal editorial teams and reviewers will carefully read and decide whether such use is permissible on a case-by-case basis. Creating, altering or manipulating original data using GenAI tools is not permitted.
COPE has also published a position statement stressing the need for transparent reporting on the use of GenAI tools. Both STM and COPE remind authors that they are fully responsible for the content of their manuscript and should carefully check any parts produced or edited by GenAI. This is largely because GenAI tools can introduce ethical issues into papers, relating to bias, plagiarism, and misinformation.
Finally, GenAI tools do not meet the requirements for authorship and should not be included as an author.
Authors should carefully consult the journal policies and recommendations on how to acknowledge and report the use of GenAI tools.
MDPI’s authorship policies
MDPI follows the ICMJE authorship guidelines while acknowledging that different disciplines adopt their own criteria. The minimum recognized requirements for authorship are in line with COPE’s recommendation:
- Making a substantial contribution to the research AND
- Accepting accountability for the work undertaken.
For complete transparency, MDPI requires that all submitted manuscripts include an author contributions statement that specifies the work of each author. This is based on the CRediT taxonomy which uses 14 key types of contributions. The 14 key contributions may not be applicable to all fields or all types of manuscripts; therefore, authors can select from the 14 criteria all those that apply to their particular case.
As all authors must approve the final version of the article and accept accountability, changes in authorship during the editorial process must be clearly justified and all authors must agree.
MDPI provides authors with an authorship change form that must be signed by all authors. Any change in authorship after publication will be investigated and if sufficiently justified, will require the publication of a correction. MDPI strongly encourages authors to carefully consider and discuss authorship in a collaborative manner before submitting the manuscript.
MDPI has also developed detailed policies regarding GenAI use in accordance with the recommendations provided by STM and COPE. Templates on how to acknowledge the use of GenAI tools and guidance on ethical use are provided.
For further detailed guidance, visit MDPI’s Research and Publication Ethics—Authorship section.










