
In Silico Docking of Bolinaquinone at Clathrin Terminal Domain
Guest Commentary by Derek McPhee
Endocytosis is a fundamental process that delivers macromolecules located outside the cell or on the cell membrane to the cytoplasm. It is a necessary process for nutrients to reach the cell, and for regulation of numerous transmembrane receptors. Of the various ways that cargo can access the cytoplasm, so called clathrin-dependent endocytosis is one of the most studied. This mechanism is generally well understood. Membrane fragments, along with their contents, enter the cell as vesicles coated with clathrin species. This activity is key to the survival and normal functioning of eukaryotic cells. It also has also been associated with a variety of pathological states. These include the access of pathogens like virus and bacteria to the cell interior, a number of cancers and other conditions.
Bolinaquinone
Bolinaquinone, is a cytotoxic marine sesquiterpene hydroquinone first isolated by de Guzman et al. in 1998 from a Phillipine Dysidea sponge, and later shown to be a clathrin inhibitor. Its relatively simple structure makes it an attractive target for systematic structural modification to enhance or change its biological activity, but details of its interaction with clathrin were lacking.
To fill this gap in our knowledge, we now have the Abdel-Hamid and McCluskey Molecules paper, “In Silico Docking, Molecular Dynamics and Binding Energy Insights into the Bolinaquinone-Clathrin Terminal Domain Binding Site”.
Docking of bolinaquinone
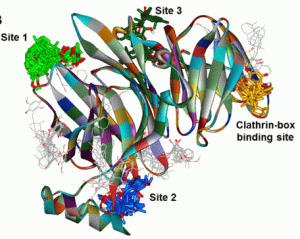
Building upon their earlier experience studying the binding site and mode of action of other small molecule inhibitors of clathrin, the authors systematically examined the docking of bolinaquinone in the clathrin terminal domain. This approach revealed that bolinaquinone appears to occupy a binding pocket distinct from that occupied by previously identified inhibitors. Exhaustive flexible docking studies and examination of the stability of docked poses and interaction energies allowed the authors to identify up to five binding sites, and propose the most likely one, thus demonstrating the flexibility and power of this virtual screening approach. The identification of the key functional group interactions should provide a most valuable guide for synthetic chemists aiming to design simplified bolinaquinone analogs to either confirm the putative binding site or as novel therapeutic agents in any of the pathologies where clathrin is known to play a key role.
References
- de Guzman, F.S.; Copp, B.R.; Mayne, C.L.; Concepcion, G.P.; Mangalindan, G.C.; Barrows, L.R.; Ireland, C.M. Bolinaquinone: A novel cytotoxic sesquiterpene hydroxyquinone from a Philippine Dysidea sponge. J. Org. Chem. 1998, 63, 8042–8044.
- Abdel-Hamid, M.K.; McCluskey, A. In Silico Docking, Molecular Dynamics and Binding Energy Insights into the Bolinaquinone-Clathrin Terminal Domain Binding Site. Molecules 2014, 19, 6609-6622.











