
Flavonoid Thiol Toxicity: What’s in a Group?
In this article, we go over recent work on thiol toxicity and antioxidants.
Antioxidants are a direct consequence of the highly oxidizing environment (21% atmospheric dioxygen) of Earth. The switch from anaerobic to aerobic metabolism during the evolution of the majority of organisms on this planet not only resulted in a more efficient liberation of energy from glucose (~30 vs. 2 ATP), but came at a price, since dioxygen has several alter egos.
With two unpaired electrons, dioxygen is a typical free radical, although that its chemistry is constrained; however, all hell breaks loose when this constraint is lifted and various highly reactive oxygen species (ROS) are formed.
Reactive oxygen species (ROS)
ROS are reactive species—many of them are free radicals. Biomolecule, especially those with oxidizable bonds or groups, are affected by them. Fortunately, nature provides a myriad of counteracting and repair mechanisms to ensure that cellular homeostasis is maintained. These include repair enzymes, antioxidant enzymes, and low molecular weight antioxidants (micronutrients), the majority of which, e.g., ascorbate, tocopherol, and beta-carotene, are taken up from foodstuffs.
Small antioxidants
Thus, a major function of small antioxidant molecules is to take away the initial “sting” of free radicals by lowering their reactivity. However, although the oxidized antioxidant’s reactivity is lower, there are antioxidant products that still react with particular susceptible biomolecules (selective reactivity and toxicity).
One group of antioxidants that display this Janus-faced property are the flavonoids. Flavonoids are ketone-containing polyphenols that are generally regarded as the primary ROS scavengers at the top of the pecking order. Oxidized flavonoids specifically targetnucleophilic thiols in glutathione (GSH) and cysteine residues of proteins, thereby impairing their biological function.
The fact that GSH is targeted is daunting, since it is like putting out a forest fire in one place, while setting fire to the same forest in multiple other places. This is because GSH is the cell’s primary redox buffer and thus the major intracellular antioxidant, which the cell is able to synthesize itself.
Thiol toxicity
In The Minor Structural Difference between the Antioxidants Quercetin and 4’O-Methylquercetin has a Major Impact on their Selective Thiol Toxicity, Lemmens and co-workers examine the structure-function relationships in antioxidant metabolites and show that the devil is in the structural detail [3], highlighting the impact that a single group can have on selectivity, reactivity, and ultimately toxicity.
To unravel the mystery behind flavonoid thiol toxicity, the authors compared two structurally related flavonoids, i.e., quercetin and tamarixetin. These only differ in a 4’O-methylated group (Fig 1).
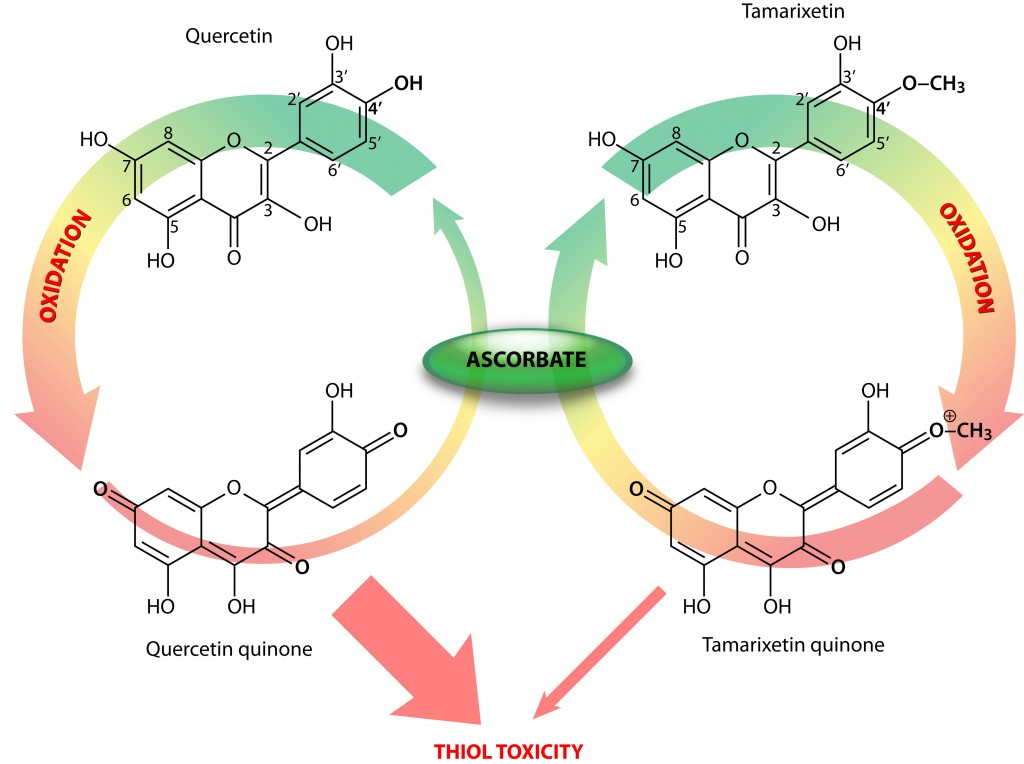
Flavonoid quinones and thiol toxicity
When they tested the reactivity of the flavonoid quinones (oxidized forms; figure above) against the thiol-containing enzyme creatine kinase (CK), an enzyme crucial for energy production in cells, with high energy turnover, they observed a significantly lower thiol reactivity and toxicity in the tamarixetin quinone. Moreover, they determined that in the presence of ascorbate, a potent recycler of oxidized antioxidants, tamarixetin quinone showed a higher preference for ascorbate than CK (Figure).
Consequently, intact tamarixetin is formed and the oxidized ascorbate in turn can be reduced by reductases that use energy-rich molecules, such as NADH, as cofactors, thereby coming full circle in the neutralization of the initiating radical.
This study points to the selectivity capabilities of these structure–function relationships in various antioxidants and intermediates formed during free radical detoxification reactions. Clearly it will be of interest to explore this also within the considerably more complex environment of the living cell.
Cited works for this article
We also encourage the reader to review these papers.
- Rich, P. R., The molecular machinery of Keilin’s respiratory chain. Biochem Soc Trans 2003, 31, (Pt 6), 1095-105.
- Skibsted, L. H., Carotenoids in antioxidant networks. Colorants or radical scavengers. J Agric Food Chem 2012, 60, (10), 2409-17.
- Lemmens, K. J.; Vrolijk, M. F.; Bouwman, F. G.; van der Vijgh, W. J.; Bast, A.; Haenen, G. R., The Minor Structural Difference between the Antioxidants Quercetin and 4’O-Methylquercetin Has a Major Impact on Their Selective Thiol Toxicity. Int J Mol Sci 2014, 15, (5), 7475-84.
Further Reading
We also refer the reader to the following.
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M., Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sci 2013, 14, (2), 3540-55.
- Cui, Y.; Han, Y.; Yang, X.; Sun, Y.; Zhao, Y., Protective Effects of Quercetin and Quercetin-5′,8-Disulfonate against Carbon Tetrachloride-Caused Oxidative Liver Injury in Mice. Molecules 2013, 19, (1), 291-305.
- Dall’Acqua, S.; Miolo, G.; Innocenti, G.; Caffieri, S., The Photodegradation of Quercetin: Relation to Oxidation. Molecules 2012, 17, (8), 8898-8907.
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A., Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers (Basel) 2010, 2, (2), 1288-311.
- Han, R. M.; Zhang, J. P.; Skibsted, L. H., Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules 2012, 17, (2), 2140-60.
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A., Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int J Mol Sci 2012, 13, (3), 3145-3175.
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T. C., Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, (10), 3779-827.
- Larson, A.; Symons, J. D.; Jalili, T., Quercetin: A Treatment for Hypertension?—A Review of Efficacy and Mechanisms. Pharmaceuticals 2010, 3, (1), 237-250.
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A., Plant flavonoids–biosynthesis, transport and involvement in stress responses. Int J Mol Sci 2013, 14, (7), 14950-73.
- Seelinger, G.; Merfort, I.; Wolfle, U.; Schempp, C. M., Anti-carcinogenic effects of the flavonoid luteolin. Molecules 2008, 13, (10), 2628-51.
- Thilakarathna, S. H.; Rupasinghe, H. P., Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, (9), 3367-87.










