Serendipitous Self-Assembly in Cluster Coordination Chemistry
Here, we explore the exciting work being done in cluster coordination chemistry on serendipitous self-assembly.
Cooperative action of transition metals
Transition metals can exhibit unique chemical, redox, magnetic or spectroscopic properties. For this reason, they play central roles in a large variety of fields, ranging from catalyzing crucial biological reactions to constituting the basis of information processing and recording. Since the pioneering work of Nobel Prize winner, Prof. Alfred Werner, at the end of the 19th century, molecular coordination chemistry has evolved from producing adducts of one central rare-earth ion surrounded by Lewis donors, to yielding giant molecules possessing dozens of transition metal centers cemented by bridging ligands.
The cooperative action of several transition metals in one molecule can yield greater benefits and is demonstrated in coordination aggregates. For example, the production of oxygen in plants occurs at a [Mn4CaO5] cluster.
Searching in the dark: Serendipitous self-assembly
These properties have stimulated synthetic coordination chemists to attempt the synthesis of polynuclear transition metal complexes. Researchers soon realized that the structural versatility of transition metals, together with the large diversity of conceivable mono- or multidentate bridging ligands, offered unlimited possibilities for the synthesis of coordination clusters and access to a variety of properties. This versatility has some drawbacks, however. It is almost impossible to predict the thermodynamic outcome from a given metal/ligand reaction system and, thus, to pre-determine the nature of the product that will result from any novel reaction. Therefore, such a synthetic method has been termed “serendipitous self-assembly”.
Serendipity in action
The search for a new generation of molecular magnets, based on high spin components, has been a major focus in this field. In one example, a paper recently published in Magnetochemistry reports the synthesis, structure and magnetic properties of a new and remarkable [Ni11] cluster (Perlepes, S. P. et al. Magnetochemistry 2016, 2(3), 30; doi:10.3390/magnetochemistry2030030).
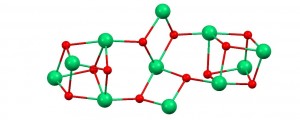
[Ni11] cluster core (Ni atoms in green and Oxygen atoms in red)
This fascinating compound is built of two lateral [Ni4O4] fragments, linked to a central [Ni3O4] unit. Basically, the oxygen atoms within these building blocks are provided by some of the bridging ligands that keep the structure together, which in turn serve to link the three parts of the assembly and provide additional bridging pathways within the molecule. The ligands that occupy the coordination sites of the metals and dictate the shape of this complex architecture are oxide, hydroxide and acetate groups, in addition to a deprotonated bis-pyridine gem-diol. The acetate groups are introduced as the counter-ions of the Ni(II) salt and the deprotonated forms of water molecules arise from the reaction solvent in the basic conditions used.
Designing the assembly
It would have been impossible to rationally designed this beautiful assembly from its components, especially considering that some of them (O2−, OH−, {(py)2(OH)O}−) generate in situ and serendipitously during the course of the reaction. However, once one such a procedure has been discovered, it can be repeated over and over again. In fact, the authors have exploited the coordination of these processes to produce a huge number of coordination clusters with almost all the transition metals of the first series, with a large number of metal centers and structures.
In their paper, the authors explain that combining this ligand with acetate in a different ratio than previously has been enough to access a completely new structural type. This gives a hint of the huge possibilities that lie ahead in exploiting this method. Like this ligand, many other families of bridging ligands have served to generate hundreds of coordination clusters through the method of “serendipitous self-assembly”, reaching sizes that have brought these molecules to straddle between the quantum and the classical world.
Controversy and outlook
Because of the unpredictable nature of this chemistry and, therefore, the lack of a very precise target, some chemists have criticized the methodology. They question whether it is truly scientific. However, there are many benefits to the approach:
- It has given access to many new coordination features and forms that could not have been imagined had not been produced accidentally. Specifically, they have helped to advance basic coordination chemistry.
- The profusion of structures and shapes has generated information of great interest to various fields. For example, in the production of extensive magneto-structural correlations (as cited in the Magnetochemistry paper [CITE]).
- From compounds obtained in this manner, important research avenues have opened up. This has been the case for single molecule magnets (SMMs) [5] and the study of the magneto caloric effect (MCE) [6] of coordination clusters.
Many scientists feel that each new molecule or structure obtained using this method is like a secret extracted from nature. Presently, it seems that there are many more secrets left to discover in this serendipitous self-assembly.










