Stem Cell Model Used to Replicate Non-Alcoholic Fatty Liver Disease For The First Time
Metabolic dysfunction-associated fatty liver disease (MAFLD), also called non-alcoholic fatty liver disease (NAFLD), affects both adults and children and is found in around 24% of the global population. A novel study published in the Open Access journal International Journal of Molecular Science by researchers from the Medical University of South Carolina found a biological pathway that could be targeted to treat the disease.
There is currently no cure for this chronic disease, which raises the risk of mortality by 70% and is the most common form of liver disease. Unlike alcoholic-related liver diseases, NAFLD is not caused by the consumption of alcohol but is characterized by the accumulation of excess fat in the liver. Several factors such as genetic predisposition, diet, and obesity contribute to the development and progression of the disease.
Non-alcoholic fatty liver disease
Disease outcomes
NAFLD is associated with many other adverse health issues, including an increased risk of cardiovascular issues, diabetes, insulin resistance and metabolic syndrome. NAFLD has different stages as it progresses. The progression of NAFLD results in hepatic fibrosis (over-production of liver scar tissue), eventually leading to cirrhosis (severe scarring). Untreated cirrhosis can lead to liver failure. With the increasing rate of NAFLD development in individuals across the globe, more research is required to understand the pathogenesis of the disease.
NAFLD disease pathway
In previous studies, a mutation in the gene called PNPLA3, which is highly expressed in the liver, has been associated with the development of NAFLD. The studies found that the mutant gene results in the production of a protein that forms abnormal interactions with lipid particles. These result in the lipids not being degraded efficiently, leading to their accumulation in the liver.
“A lot of patients with fatty liver have this (PNPLA3) mutation, and we don’t know what it’s doing or why it’s increasing susceptibility to fatty liver,” said Doueiry, lead author of the article.
In the first study of its kind, Douiery and their research team used a human stem cell model to recapitulate the onset of NAFLD. Previously, only mouse models were used. Although biologically similar, and serving as excellent models, there were still apparent differences in NAFLD disease manifestations in the mice. Therefore, the study explores the use of human stem cells to better model the disease. It also aims to identify specific biological pathways which can be targeted for treatment.
Using a stem cell model to identify key pathways
Proving pluripotency
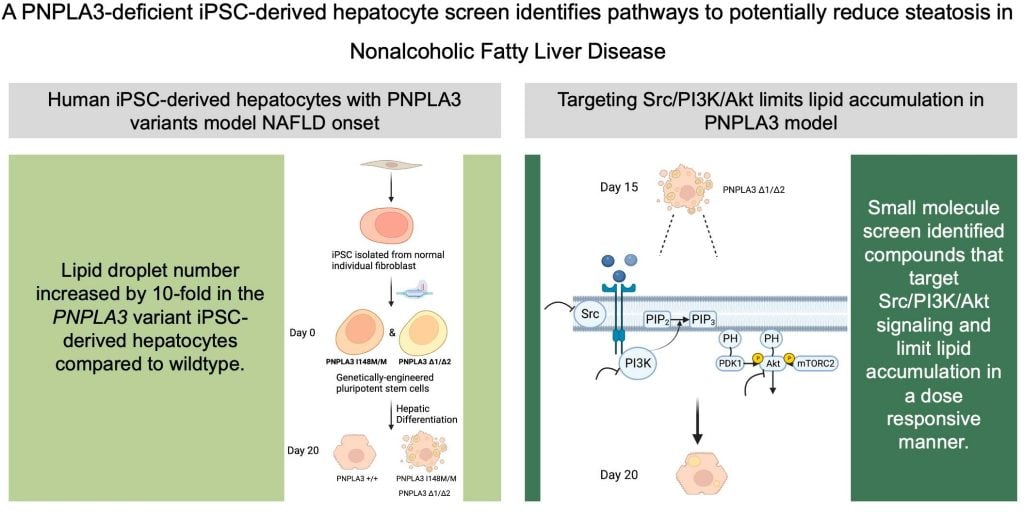
The researchers used induced pluripotent stem cells (iPSCs) derived from hepatocytes (liver cells) to model NAFLD. Here, they generated iPSCs with a variant of the PNPLA3 gene (PNPLA3I148M/M ) or with a disrupted PNPLA3 gene function (PNPLA3∆1/∆2). They developed this model through the use of the genome engineering tool CRISPR-Cas9. This tool enables researchers to make genetic modifications to cells to generate genetic variants. The researchers then ensured the iPSC displayed clear pluripotency markers (biomarkers that confirm the cells are pluripotent) and that they were able to differentiate into hepatocyte-like cells.
Looking at lipid levels
Following this, they measured and analysed the lipid levels in both PNPLA3I148M/M and PNPLA3∆1/∆2
cell populations. Here, they found that the lipid levels in both variants were significantly elevated. This aligns with previous findings of NAFLD etiology, in that lipid accumulation from PNPLA3 malfunction results in the manifestation of the disease.
Here, Dr. Douneiry reflects on their findings:
“I knew that the mutation in humans was causing the fat accumulation, but I had no idea what I was going to see in the plate,” she said. “Looking at the liver cells carrying the genetic mutation for the first time was so exciting because I knew we had a model that we can use to find some answers.
Screening for a small molecule for liver disease
As the researchers saw that the iPSC model successfully modelled NAFLD, they then used these cell populations to test various drugs as a potential treatment for the disease. They used the Tocriscreen Mini library consisting of 1120 compounds that are known to be involved in specific parts of the lipid regulatory pathway. They tested each of the drugs on the mutant iPSC cell population, to see what would happen.
Here, they were able to identify molecules that inhibited the Src, PI3K and Akt signalling pathways and reduced lipid accumulation. These pathways are also key in cell cycle progression, proliferation and growth.
“With these results, we knew we weren’t just looking at random molecules doing something. The fact that they all connected through a pathway showed us that we were onto something,” – Dr. Douneiry
The research team found five compounds that were able to reduce lipid accumulation through inhibition of the mentioned cellular pathways without significantly affecting cell survival. This means that the drugs could act as a potential therapeutic avenue for the treatment of NAFLD.
Modelling non-alcoholic fatty liver disease and more
Dourier’s novel study has proven that it is possible to use iPSCs to model not only NAFLD but other genetic diseases which require further research.
“Caren’s study has shown that human stem cell-derived liver cells with MASLD mutations can be used effectively to identify pathways that can be targeted by drugs to reduce fat levels in the liver,” – Stephen Duncan, Endowed Chair in Regenerative Medicine at the Medical University of South Carolina
Some of the drugs that were seen to reduce lipid accumulation in the iPSC model are either already FDA-approved or in clinical trials for breast cancer treatment. Knowledge of the drugs could expedite their use for NAFLD treatment. However, much more in vitro (research outside of a living organism) and in vivo research (research within a living organism) is required to fully understand their mechanism of action and effect on lipid accumulation in the NAFLD disease state.
Nonetheless, the promising results shown in the study show exciting avenues for NAFLD treatment. The findings give a robust foundation for further research in NAFLD with the use of iPSCs as a model, as well as the use of various small molecules in reducing lipid accumulation. It also gives hope for those suffering from NAFLD of a potential cure for the debilitating disease.
To learn more about research like this, access MDPI’s Open Access journal IJMS, or have a read of the Special Issue: Recent Research on Stem cells to Organoids.










